Drinking water issues
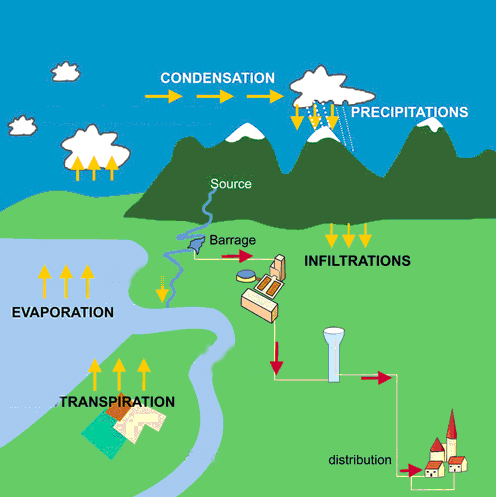
Water cycle.
Fullerenes (C60) for example, are generally considered not a lot soluble in water. But in some pH conditions they reacts with water to give colloidal aggregates of diameters between 25 and 500 nm that are quite a lot more soluble (1000 mg/liter) and relatively stable (15 weeks) in fresh water. So certain hydrophobic particles can be potentially very mobile in the environment.
What is nanoparticle mobility in surface and subterranean waters?
How aggregation and sorption can modify nanoparticle transport?
What characteristics affect their biological availability?


