Food additives issues
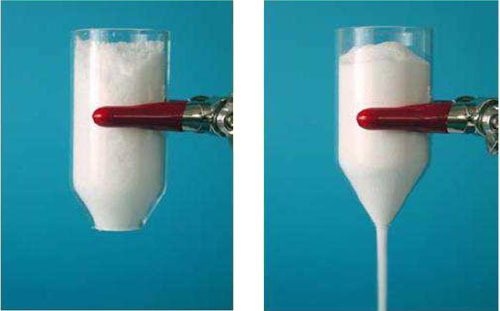
Sea salt BEFORE and AFTER silica SiO2, E551 addition.
The necessary studies of harmlessness for a request of authorization of launch on the market of a food additive cover all the aspects of the toxic kinetics, the metabolism, the toxicology and the tolerance at the man. Specific criteria of purity of additives can be resumed in directives 95/31/CE, 95/45/CE and complementary 96/77.
For some products only (microcrystalline cellulose, carraghenane, arabic gum) the size of particles is the object of specific statutory criteria. No more than 10 % of particles microcrystalline cellulose does not owe for example, to have a size lower than 5 micrometers).
Modification of the absorption with the other nutriments?
Modification of biodistribution?
Evaluation of the contributions towards the limits of daily contribution?
Physical characteristics and toxicity of additives?


